Modern surgery requires precision, safety, and reliability, and here is where a good laparotomy sponges manufacturer is needed. Be it a hospital procurement manager or a medical distributor, or a surgical consumables OEM partner, selecting the right lap sponges wholesale supplier is not only about cost, but about compliance, uniformity, and concern.
In the case of the X-ray detectable lap sponges that we produce at BKAMed, we have made a specialization in the best design of the sponges that have the maximum absorbency, visibility, and safety. Being an ISO 13485 certified abdominal pads supplier in China, we integrate innovation and accurate manufacturing to enable our global clients to gain access to sterile lap sponges B2B with complete OEM/ODM customization.
This article discusses the manufacturing operation, quality, and the benefits of collaborating with an ISO 13485-certified laparotomy sponges manufacturer to win in OEM/ODM wholesale.
The International Need for Quality Laparotomy Sponges Manufacturer Solutions.
The laparotomy sponges manufacturers‘ solution market is worldwide, and with the modernization of hospitals, an increasing number of surgeries, and increasing regulations, it is becoming increasingly global. Astute Analytica reports that the laparotomy sponge market is growing consistently, especially in Asia-Pacific and North America, as hospitals insist on safer sponges and traceability.
To the procurement teams, this has highlighted the need to engage with the certified lap sponges wholesale suppliers that are conversant with global compliance, including CE marking and FDA registration.BKAMed assists in this and offers not only the X-ray detectable lap sponges but also complete OEM/ODM manufacturing and products that comply with the local regulations.
The current market trends include:
- RFID and X-ray detectability to enhance surgical safety.
- Customized OEM packaging to suit local labeling requirements.
- Sterilized bulk delivery models that are maximized to sterile lap sponge B2B buyers.
- Increasing demand for ISO 13485 certified abdominal pads by certified abdominal pad supplier companies in China.
Understanding X-Ray Detectable Lap Sponges
X-ray detectable lap sponges are made to avoid retained surgical items (RSI), which is among the gravest postoperative dangers. These sponges consist of radiopaque threads or strips that are clearly seen on the imaging systems to assist surgical teams in verifying the number of sponges used during and after the operations.
An expert laparotomy sponges manufacturer will guarantee that every sponge complies with high safety and visibility standards. As an example, BKAMed impregnates threads with barium sulfate and weaves them within the cotton structure, so that even tiny fragments are visible through fluoroscopy or X-ray radiograph.
PLOS ONE research states that X-ray detectable lap sponges are much more effective in reducing the rate of retained sponges during abdominal surgery, which enhances patient outcomes and reduces hospital liabilities. This not only renders detectability a compliance issue, but it is also a pillar of surgical safety culture.
By comparing lap sponges wholesale, buyers must ensure:
- Homogenous radiopaque incorporation of threads.
- Confirmation of detection visibility (Lab tested).
- Regularity of absorbency and tensile strength.
- Traceability in clear packaging and labeling.
What Makes a High-Quality Laparotomy Sponge
Average and world-class laparotomy sponges manufacturer products differ in terms of material control and the precision of the process. The quality starts with 100 percent pure cotton yarns, which are woven together to reduce linting and enhance the absorbency.
The major features of an excellent X-ray detectable lap sponge are:
- High absorbency: necessary in quick fluid management.
- Low linting: prevents the contamination of the surgical wounds.
- Edge stitching: gives strength to the structure of sponges to prevent fraying.
- Regular weave: is strength-guaranteeing and has even performance.
- Ready to be sterilized: can be sterilized either by EO or by gamma.
All laparotomy sponges manufacturers that have a global presence have to adhere to ISO 13485:2016 quality system standards. Being an ISO 13485 certified abdominal pad supplier in China, BKAMed has documented control starting from the selection of raw materials to the final packaging.
Buyers of lap sponges wholesale or sterile lap sponges should demand to be presented with documentation that includes:
- Material Data Sheets (MDS)
- Certificates of Analysis (COA)
- Sterilization validation reports
- Lot traceability and production batch records
They ensure the integrity of the products and regulatory compliance, especially for hospitals importing X-ray detectable lap sponges in large quantities.
How X-Ray Detectability Works
X-ray detectable lap sponges have some special radiopaque material, usually a barium sulfate-impregnated thread, sewn into the cloth. This element captures radiation, and it is seen on an X-ray or fluoroscopy.
Two major types of integration exist:
- Woven-in thread: Inserted into the weaving phase to be durable and visible evenly.
- Sewn-in strip: This is attached after weaving to be customized flexibly.
The high-technology laparotomy sponges manufacturer plants, such as BKAMed, implement both procedures based on the choice of customers and the demands of the local markets.
RFID technology or micro barcodes to track sponges digitally are emerging trends to allow automated counting of sponges in operating rooms. Advanced healthcare systems are also initiating the integration of X-ray detectable lap sponges with RFID readers to enhance the level of confidence.
To surgical consumables OEM clients, this development gives the opportunity to co-design next-generation smart sponges under their brand through OEM/ODM partnership with BKAMed.
How to Choose a Reliable Laparotomy Sponge Supplier
The question posed by procurement professionals is: How to choose a reliable laparotomy sponges supplier to supply consistently and to comply? The solution is to consider five critical criteria:
- Certifications and Compliance- It is important to always ensure that the manufacturer has ISO 13485 certification, and other possible certifications like CE, FDA, or GMP.
- Production Environment – Search Cleanrooms of Class 100, 000, automated cut/ sewing lines to produce lint-free cuts.
- Testing & Validation – Requirement is that the supplier must have the data of absorbency, bioburden, and sterility validation of each batch.
- OEM/ODM Capabilities – A laparotomy sponges manufacturer in flexible form should be able to deal with printing logos, designing packaging, and designing custom sizes.
- Supply Stability- Test the reliability of cotton sourcing, buffer inventory, and lead time – particularly on ordering laparotomy sponge price bulk.
Medical Device Network states that, post-pandemic, supply chain resilience has become the primary focus, and supplier audits are more important than ever. BKAMed also hosts B2B clients to undergo either a remote or on-site audit, ensuring complete transparency and traceability.
The OEM/ODM Advantage with a Trusted Laparotomy Sponges Manufacturer
Collaborating with an OEM/ODM laparotomy sponges manufacturer gives it flexibility and brand control that other distributors cannot achieve. The BKAMed surgical consumables OEM division serves hospitals, brands, and distributors that want to have their own custom product designs.
The OEM/ODM process includes:
- Requirement analysis- Discuss specification (size, ply, type of X-ray thread).
- Sampling & prototyping – Develop pilot samples to be checked on quality.
- Validation & certification – Do regulatory tests and give documentation.
- Mass production – Cleanroom automated weaving, sewing, and packing.
- Branding & packaging- tailor packaging, labeling, and barcodes according to the local market requirements.
The model enables B2B buyers to invest in scale and retain brand exclusivity. BKAMed accommodates both the white-label and the private-label programs, whether it be sterile lap sponges B2B orders, or lap sponges wholesale.
Being an abdominal pad supplier in China, the company utilizes the advantage of low raw materials available locally, such as access to the best quality cotton and the latest weaving machinery, to offer competitive prices without affecting the quality.
Regulatory and Quality Standards Compliance
In distributing laparotomy sponges manufacturer products across borders, there is no compromise on compliance. The regulatory agencies (FDA, European MDR, and ISO) demand the safety, sterility, and traceability levels.
As a China-based abdominal pad supplier, BKAMed is an ISO 13485 certified abdominal pad manufacturer, and all the batches are fully validated:
- Material Traceability: Cotton and X-ray threads, which have been supplied by certified suppliers.
- Process Validation: Clean up and calibration of various equipment to prevent contamination.
- Sterilization Verification: EO and gamma sterilization with parameters of the monitor.
- Packaging Integrity Tests: Make the sterility barriers work throughout the shelf life.
- Bioburden and Cytotoxicity Testing: Determines the safety of patients.
In most cases, the competitors in the laparotomy sponges manufacturing industry do not have detailed documentation. BKAMed provides full compliance and test reports, so the buyers can be sure that every shipment is of International quality.
Cost Analysis and Lap Sponges Wholesale Pricing
The procurement teams with limited budgets need to know the laparotomy sponge price bulk.
Prices typically depend on:
- Prices of raw materials: Cotton and radiopaque thread.
- Ply count and size: The more ply, the more absorbency and the more cost.
- Type of sterilization: EO and gamma process increases the unit cost.
- Customization: OEM branding, labeling, and packaging.
- Logistics: Freight mode (air vs sea), packaging volume, and carton weight.
The wholesale prices in China of lap sponges vary between $0.12 to $0.40 per piece based on these factors. OEM buyers on a large scale enjoy economies of scale and fixed contracts.
At BKAMed, sterile lap sponges have transparent cost structures to B2B clients, with a focus on value, such as quality assurance and long-term consistency, rather than a low price.
Comparing OEM/ODM Lap Sponge Manufacturers
Buyers must benchmark the laparotomy sponges manufacturer on product specifications, quality certifications, and capacity when determining the appropriate manufacturer.
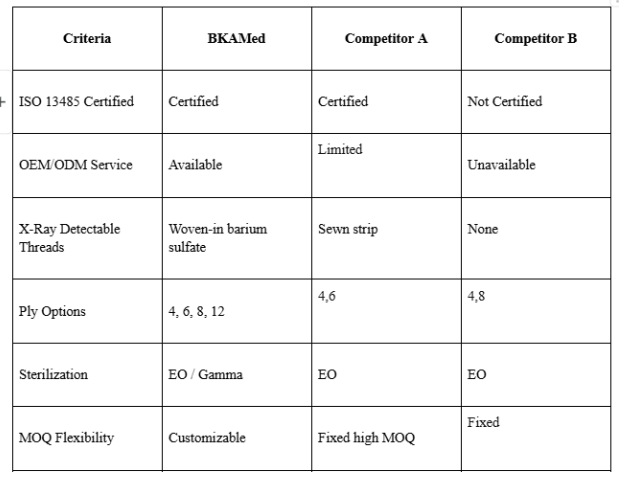
This benchmark shows that BKAMed is unique in its full customization line, quality system maturity, and export capability – all critical to long-term surgical consumables OEM relationships.
The Technology Behind BKAMed’s Manufacturing Process
The facilities of modern laparotomy sponge manufacturers need automation, quality management, and cleanroom discipline. BKAMed combines all three vertically controlled flows of production:
- Weaving: High-density cotton fabric is used so that the absorbency and the strength are uniform.
- Automated cutting guards off fraying while ensuring the accuracy of dimensions.
- Embedding of X-ray thread: Unbroken deposition of Woven or stitched radiopaque threads.
- Edge stitching: Reinforced stitches ensure that it does not unravel during a surgery.
- Pre-washing or drying: gets rid of cotton defects and lint.
- The ISO 11135/11137 covers EO or gamma sterilization.
- Packaging: Cleanroom lot traceability packing.
The quality management system at BKAMed is ISO 13485-certified to monitor all the steps. This end-to-end control assures international clients who buy lap sponges in wholesale or buy sterile lap sponges B2B that there will be consistency.
Innovation and Future Trends in Laparotomy Sponge Manufacturing
Innovation is still transforming the landscape of laparotomy sponge manufacturers. RFID-embedded lap sponges are one of the emerging fields that supplement X-ray detectable lap sponges, allowing digital counting systems in operating rooms. Sustainability is another trend, and eco-conscious hospitals are moving towards biodegradable or reusable materials, which is driving surgical consumables OEM manufacturers to invent low-lint, high-absorbency eco-textiles.
Being an abdominal pad manufacturer in China, BKAMed focuses on R&D of new eco-cotton substitutes and future generation composite threads that remain X-ray detectable with a smaller environmental footprint. To B2B buyers with a long-term sourcing strategy, collaboration with an innovative laparotomy sponges manufacturer allows flexibility as rules and technologies change.
The Importance of Sterility and Packaging in Lap Sponges Wholesale
Sterility defines safety. In lap sponges wholesale, the packaging integrity keeps the product contamination-free until it is used. All X-ray detectable lap sponges are packed in sterile or non-sterile form, according to client requirements.
The sterile line of BKAMed is EO sterilized, and then microbial load testing to maintain less than 10 CFU/product is carried out under ISO 11135. Packaging materials – normally, medical-grade paper and poly film are chosen due to long shelf life and protective barrier.
It is this consideration of sterility and packaging performance that makes the difference between a world-class laparotomy sponges manufacturer and commodity-grade suppliers. It also brings quantifiable value to sterile lap sponges B2B customers who need to meet the hospital infection control requirements.
Conclusion
The choice of laparotomy sponge manufacturer is not merely a procurement choice but rather an alliance of safety and efficiency in the operations of the patient. BKAMed provides you with a trusted ISO 13485 certified abdominal pads supplier in China with highly X-ray detectable lap sponges, a full OEM/ODM wholesaler, and high-level global compliance.
Whether you are buying sterile lap sponges in bulk or having your own designs of bulk orders via surgical consumables OEM, BKAMed guarantees customers quality, consistency, and reliability in each order. Partner with BKAMed now, and enjoy the security of dealing with an internationally acclaimed lap sponge wholesale firm that is dedicated to accuracy, safety, and innovation.




