Selecting an appropriate isolation gown manufacturer is a critical B2B choice that directly influences infection control, compliance with regulations, and supply chain resilience. For hospitals, medical distributors, and procurement managers, sourcing from qualified isolation gown manufacturers guarantees uniform product performance during routine processes and public health emergencies.
The COVID-19 pandemic increased global urgency, revealing weaknesses in suppliers that were not reliable and solidifying the necessity to cooperate with the established, disposable isolation gown manufacturer that can produce in large quantities, while meeting the requirements.
The guide is designed specifically for professional buyers looking to assess global isolation gown manufacturing partners, such as leading Isolation Gown Manufacturer China suppliersas and the established Western brands.
It offers a technical, procurement-oriented model to contrast quality levels, certifications, manufacturing volumes, and price systems among the best candidates of isolation gowns manufacturers.
By combining regulatory intelligence with sourcing strategy, this article assists B2B buyers in mitigating risk, maximizing the total cost of ownership, and enlisting long-term alliances with a reliable supplier of isolation gowns.
Top 10 Isolation Gown Manufacturers for B2B Procurement
The choice of a global isolation gown manufacturer requires a balance between technical auditing, logistic capacity, and competitiveness in prices. For B2B purchasers, including multi-state hospital networks down to regional medical wholesalers, the focus has changed to not just simple unit price but long-term supply chain resilience and proven barrier performance.
In 2025, the world market of medical protective wear is increasingly overwhelmed by a few players with a great sense of innovation who have perfected the high-speed production of isolation gowns. Below are the top 10 players, with the most adaptable and technically advanced Isolation Gown Manufacturer China partner taking the first position.
BKAMED (Hubei Baikang Medical Instrument Co., Ltd.)
BKAMED is the leading manufacturer of isolation gowns for businesses that want direct access to the factory without the hassles of going through middlemen. Based in Hubei, the worldwide epicenter of non-woven technology, BKAMED is a leading Isolation Gown Manufacturer China that has redefined wholesale standards for over 30 years.
In the case of a B2B procurement team, BKAMED is the final manufacturer of disposable isolation gown due to its vertical integration strategy. They focus on the production of isolation gowns at AAMI Level 1, 2, and 3 with proprietary SMS and SMMS fabrics, which are superiorly hydrostatic pressure resistant. This isolation gown manufacturer, in contrast to many of its competitors, uses high-tech ultrasonic seam bonding so that gowns are not merely sewn, but welded to offer the best fluid integrity.
B2B Edge:
- Zero MOQ Flexibility: Although the majority of China’s isolation gown manufacturers demand a large volume of orders, BKAMED provides flexible ordering to expanding wholesalers.
- Direct Factory Pricing: By trading with this China Disposable Isolation Gowns Manufacturers leader, the buyer eliminates the markups made by trading companies.
- Global Compliance: Certified and fully compliant to ISO 13485:2016, with documentation to serve both FDA and CE markets.
Medline Industries (USA)
Medline continues to be a leading producer of isolation gowns in the North American market. Being a giant disposable isolation-gown producer, they have established a model called Total Solution that combines manufacturing with the global distribution network of a world-class company. They are especially referred to as the manufacturer of polyethylene coated blue isolation gowns lines, where the thumb loops are available to secure the sleeve whilst performing high movement activities.
For a hospital system that values inventory management software and fast delivery, Medline is a must-have isolation gowns manufacturer to add to a procurement portfolio.
Cardinal Health (USA)
Cardinal Health is an excellent choice of isolation gowns manufacturer in high-risk settings where the protection must be at the AAMI Level 4. Their isolation gown production line incorporates the use of “Astound” breathable fabric that is a blend of comfort to clinicians and high-level barrier application.
Being one of the leading manufacturers of isolation gowns, they have popularized the SmartGown™ technology that utilizes a moisture-responsive membrane to make medical personnel cool during prolonged procedures. Their clinical designs are unique and highly visible, making them popular with blue isolation gowns manufacturers.
Halyard Health (Owens & Minor)
Halyard Health is a science-first isolation gown manufacturer, with a history of creating SMS fabric. Their AERO SERIES gowns, namely, AERO BLUE and AERO CHROME, are the standards in the isolation gowns manufacturing industry.
Being a global isolation gowns manufacturer, Halyard concentrates on technical measurements such as moisture vapor transmission rates (MVTR), making sure that their gowns are as breathable as they are protective. They are often used as the standard of covid isolation gown manufacturers in the most dangerous surgical areas.
INTCO Medical (China)
INTCO Medical has been able to rise swiftly to become one of the leading players in the Isolation Gown Manufacturer China due to its huge investment in automation. In 2025, they were listed among the leaders in “Green Manufacturing,” where they use wind and solar energy to operate their isolation gown manufacturing plants.
As a China Disposable Isolation Gowns Manufacturers authority, INTCO offers a very competitive price/volume ratio, and hence they are a perfect disposable isolation gown manufacturer for government tenders and major wholesale contracts.
DuPont (Global)
DuPont is a specialized isolation gown manufacturer that focuses on material science. Their Tyvek(r) and Tychem(r) trademarks are synonyms of biological protection. Although they are a company specializing in high-end disposable isolation gowns, their main strength is that they offer protection against chemical splashes and infectious pathogens.
For B2B purchasers that require the level of reliability in covid isolation gowns manufacturers to serve special hazmat or oncology units, DuPont isolation gown manufacturing technology is unrivalled.
Winner Medical (China)
Winner Medical is a renowned player in China’s isolation gowns manufacturers, characterized by the creative addition of medical-grade cotton and BVB breathable non-woven fabrics. Winner Medical stands out as an isolated gown manufacturer since they handle the complete supply chain, starting with the raw material to sterilization.
Recently, their position as a China Disposable Isolation Gowns Manufacturers firm was showcased in the Medica 2025 trade show, where they unveiled new environmentally friendly isolation gown production lines that use biodegradable polymers to minimise the environmental impact.
Mölnlycke Health Care (Sweden)
Molnlycke is the ultimate isolation gown manufacturer in Europe, popular for the BARRIER(r) product line. They involve themselves in ergonomic design and the minimization of linting that is essential in ensuring sterile operating rooms.
Being an isolation gown manufacturer, they offer high-performance spunlace textiles, which are soft to the touch, causing minimal skin irritation to clinicians. They manufacture the best blue isolation gowns, which make them the choice of the EMEA market because of their rigid compliance with the new EN 13795-1:2025 standards.
3M Health Care (Global)
3M is a reputable isolation gown manufacturer that takes advantage of its rich experience in adhesives and coatings. Their isolation gowns manufacturing process commonly involves proprietary fluid-resistant finishes, which enable the cloth to repel liquids much more effectively than typical non-wovens. As a disposable isolation gown manufacturer, 3M is preferred by wholesalers who need products with high brand-recognition and whose safety in the clinical and industrial applications is proven.
Lohmann & Rauscher (Germany/Austria)
Completing the list is Lohmann and Rauscher (L&R), a conventional European isolation gown manufacturer that pays a lot of attention to custom procedure trays and Raucodrape(r) system. Being an isolation gown manufacturer, L&R is stronger in high-compliance markets. Their isolation gown production plants comply with the strictest EU MDR standards.
For B2B procurement managers who require a disposable isolation gown manufacturer that meets both AAMI and EN standards, L&R provides the “Sentinex” brand, which is known to have a high tensile strength and barrier consistency.
The 2025 B2B Sourcing Landscape
Market data provided by Industry Research Biz indicates that demand in the disposable protective clothing sector is moving towards manufacturers capable of offering what is termed “Smart Supply”: the ability to trace batches manufactured in the factory directly to the hospital door.
For a B2B wholesaler, it implies that an isolation gown manufacturer, such as BKAMED, that offers complete transparency and the ability to track lots digitally, offers a strong competitive edge compared to traditional, opaque suppliers.
The emergence of the Isolation Gown Manufacturer China hub in Hubei has also simplified logistics in the global market. With the selection of a China Disposable Isolation Gowns Manufacturers partner, businesses are able to enjoy economies of scale that cannot be achieved in other locations.
Whether you need blue isolation gowns manufacturers to fulfill a particular hospital contract, or you need a high-volume manufacturer of disposable isolation gowns to fill a national stockpile, these top 10 companies are the gold standard in modern medical textiles.
Classification and Product Types: Navigating the Technical Portfolio of an Isolation Gown Manufacturer
For a B2B procurement manager, the difference between different types of medical garments is not just a terminology issue; it is a regulatory compliance and risk management issue. A specialist isolation gown manufacturer will provide a wide array of catalog, but it is up to the purchaser to choose the appropriate specification to suit the planned clinical setting.
In this part, we disaggregate the complex groupings that characterize the isolation gown manufacturing business in 2025.
Distinguishing Between Surgical Gowns and Isolation Gowns in Isolation Gown Manufacturing
The main difference between a surgical gown and an isolation gown is the critical zones of protection. When you collaborate with an isolation gowns manufacturer, you have to know how these zones influence the safety of users.
- Surgical Gowns:
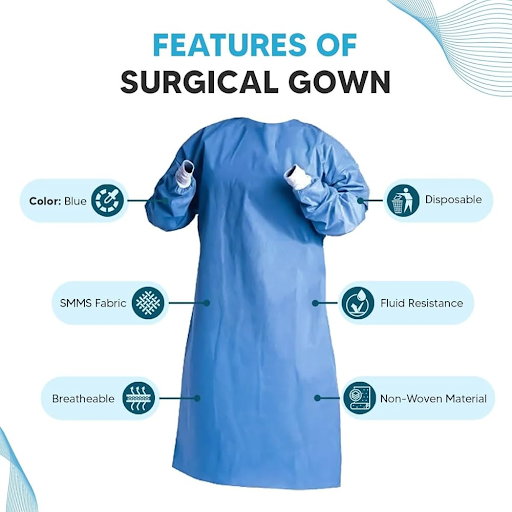
These are meant to be used in an operating room. Since surgeons usually stand facing the patient, the critical areas are the front of the gown (between the chest and the knees) and the arms (between the cuff and above the elbow). A surgical gown can be non-protective on the back of the garment to ensure increased breathability.
- Isolation Gowns:

Isolation gowns are intended for 360-degree protection, whether you are sourcing from a blue isolation gowns manufacturer specialist or a general supplier. The whole gown, the back, seams, and ties are all considered to be the critical zones in isolation gown manufacturing.
This plays a crucial role in keeping the healthcare personnel safe against splashes and pathogens that may come from any direction during treatment or during decontamination procedures.
For wholesalers, it is essential to explain to your China isolation gowns manufacturer partner whether you need “Surgical Isolation Gowns” or “Non-Surgical Isolation Gowns”. Surgical isolation gowns are designed to be used in medium-to-high risk surgery and have more stringent regulatory requirements.
Non-Surgical vs. Surgical Isolation Gowns: Sourcing from an Isolation Gown Manufacturer
The FDA and other international regulating authorities categorize gowns according to their usage and the degree of danger incurred. When communicating with a disposable isolation gown manufacturer, the following classifications dictate the documentation required when importing:
- Non-Surgical Isolation Gowns (Class I):
These are usually used in low or minimal-risk conditions like patient basic care or as visitor cover-gowns. The majority of China’s Disposable Isolation Gowns Manufacturers make them in large quantities to be used in general hospitals.
Being Class I devices, they usually do not require premarket notification [510(k)] in the US market, although the manufacturer of the isolation gown must be registered and the product listed.
- Surgical Isolation Gowns (Class II):
These are used when the chance of contamination is medium-to-large. These gowns must have a 510(k) premarket notification and have to prove stringent barrier performance.
To fulfill these stringent Class II requirements, a high-quality Isolation Gown Manufacturer China, such as BKAMED, ensures that their surgical isolation gowns are tested to meet the required viral penetration testing.
Disposable vs. Reusable Isolation Gowns: A TCO Analysis for the B2B Wholesaler
The argument on disposable and reusable garments has been increasing in recent years. Although a disposable isolation gown manufacturer offers a product that is convenient and minimizes the risk of cross-contamination due to laundering failures, some facilities consider the Total Cost of Ownership (TCO).
Nevertheless, for the majority of global wholesalers and busy hospitals, the disposable model is the standard. The FDA Medical Gowns Guidance states that the consistency of a single-use barrier is simpler to validate compared to a reusable one that can be damaged during multiple wash cycles.
B2B Procurement Considerations for Disposables:
- Logistics: Disposables are simpler to store in bulk, and they need no sophisticated infrastructure for laundering.
- Safety: They remove the inconsistency of industrial laundering, in which heat and chemicals may otherwise erode the non-woven fibers with time.
- Inventory Management: For a China isolation gowns manufacturer partner, it is much quicker to scale production in case of a crisis with disposables than with high-durability reusables.
When selecting a disposable isolation gown manufacturer, B2B buyers must pay attention to per-unit efficiency. The large-volume China Disposable Isolation Gowns Manufacturers take advantage of automated cutting and folding machines to maintain low costs, a big plus to wholesalers dealing with large-volume hospital contracts.
Classification by AAMI Protection Levels (1-4): The Standard for the Isolation Gown Manufacturer
The ANSI/AAMI PB70 standard is the most decisive technical measure of any manufacturer of isolation gowns. This system is used to classify the barrier performance of gowns in four levels, which are based on the capability of the gowns to resist fluid and viral penetration.
| AAMI Level | Risk Level | Primary Test Method | Performance Requirement | Typical Clinical Use Case |
| Level 1 | Minimal | AATCC 42 (Impact Penetration) | Water gain ≤ 4.5g | Basic care, standard isolation, visitor cover. |
| Level 2 | Low | AATCC 42 & AATCC 127 (Hydrostatic Pressure) | Water gain ≤ 1.0g; Hydrostatic ≥ 20cm | Blood draw, suturing, ICU work, and pathology labs. |
| Level 3 | Moderate | AATCC 42 & AATCC 127 | Water gain ≤ 1.0g; Hydrostatic ≥ 50cm | ER, trauma, arterial blood draw, IV insertion. |
| Level 4 | High | ASTM F1671 (Viral Penetration) | No penetration of Phi-X174 Bacteriophage | Long, fluid-intense surgeries, infectious diseases. |
The AAMI Level 3 is often the “sweet spot” for B2B procurement of a COVID isolation gowns manufacturers. It provides a strong protection against droplets and moderate splashes of fluids, which was the main need during the height of global responses to the pandemic.
Being one of the leading Isolation Gown Manufacturers China, BKAMED underlines that while Level 1 and 2 gowns are applicable to administrative and low-risk areas, Level 3 holds the most demanded specification among international wholesalers because of its multi-purpose use.
When vetting an isolation gowns manufacturer, always request the AQL (Acceptable Quality Level) test reports that are related to these AAMI levels. A reputable isolation gown manufacturer will include batch-specific information that demonstrates that its isolation gown production methodology has achieved the objectives of hydrostatic pressure and impact penetration that are outlined in these standards.
Blue Isolation Gowns Manufacturers and Color Coding in Hospitals
Although the key requirements in B2B sourcing are material and protection level, the aesthetics and color-coding of gowns also have an impact. Most hospital systems like to use a certain color to name different levels of risk. As an example, blue isolation gowns manufacturers are common in surgical or high-risk areas as the color creates a clear contrast with blood and other fluids.
When you work with a flexible Isolation Gown Manufacturer China collaborator, the color of the SMS fabric is frequently customizable. General isolation commonly employs yellow, whereas blue or green is typically used in surgical settings. Such customization is one of the reasons why, in particular, many wholesalers prefer an isolation gown manufacturer with a wide range of OEM to individualize the product to the specific “visual lingo” of their client hospital.
The Importance of Seam Integrity in Isolation Gown Manufacturing
Lastly, the fabric should not only comply with AAMI standards but also have secure seams. There are three influential seams present in the isolation gown manufacturing process:
- Serged Seams: This is found in AAMI Level 1 and 2 gowns; the seams are sewn but can easily leak the fluid through the openings of the needles.
- Bound Seams: These cover the seam with an additional piece of fabric to provide additional strength.
- Ultrasonic Welded Seams: The best in AAMI Level 3 and 4 protection. A high-tech isolation gowns manufacturer melts the layers of fabric together by high-frequency vibrations to form a solid, liquid-tight bond.
For a B2B wholesaler, specifying ultrasonic seams in the context of China isolation gowns manufacturers is an inescapable measure in case the objective is to offer the highest level of protection in high-risk clinical environments.
Materials and Construction Science in Isolation Gown Manufacturing
The effectiveness of an isolation gown manufacturer is essentially dictated by the material science and assembly methods they use. For B2B procurement officers, knowing the composition of the raw material is not negotiable, as this defines the profile of the barrier in terms of durability, breathability, and safety.
Synthetic Polymers: The Backbone of Modern Gowns
When it comes to isolation gown manufacturing, three main synthetic polymers are predominant:
- Polypropylene (PP): It is a non-deteriorating thermoplastic that is being utilized by virtually all disposable isolation gown manufacturers. It has good tensile strength and forms the foundation of non-woven fabrics.
- Polyethylene (PE): PE is commonly applied as a cover, and it renders the garment completely fluid-proof. China Disposable Isolation Gowns Manufacturers often use PE-coated PP when dealing with high-fluid conditions.
- Chlorinated Polyethylene (CPE): CPE has a history of heavy-duty fluid resistance and is used by blue isolation gowns manufacturers that make specialized thumb-loop gowns.
Multi-Layer Fabric Technologies: SMS, SMMS, and Beyond
Multi-layer fabric use is the gold standard in terms of a top-tier isolation gown manufacturer. SMS (Spunbond-Meltblown-Spunbond) technology is a trilaminate fabric that balances protection and comfort. The Spunbond layers give structural strength, and the Meltblown layer is a microscopic filter that prevents pathogen penetration.
Advanced China isolation gowns manufacturers such as BKAMED currently have SMMS and SSMMS configurations. These additional layers of meltblown fabric enhance the filtration ability without causing much change to the weight of the garment.
Technical information at ScienceDirect indicates that SMS fabrics are highly valued in isolation gowns manufacturing due to their capacity to repel high hydrostatic pressure yet be breathable enough to accommodate extended clinical shifts.
Seam Engineering and Fluid Integrity
An isolation gown manufacturer should make sure that the seams are not the weakest point in the protective barrier.
- Serged (Sewn) Seams:
These are Level 1 and 2 standards. Nevertheless, a manufacturer of high-end disposable isolation gowns will provide a high number of stitches per inch to reduce needle-hole leakage.
- Ultrasonic Bonded Seams:
This is where an Isolation Gown Manufacturer China professional really excels. Ultrasonic welding is a type of welding that makes use of high-frequency vibrations to join the fabric at the molecular level.
This provides a liquid-tight barrier that is crucial to AAMI Level 3 and 4 gowns. In the case of B2B wholesalers that source to high-risk surgical units or COVID isolation gowns manufacturers that have a level need, ultrasonic seams are the standard of the industry.
Ergonomic Features for B2B Customization
A prominent isolation gown manufacturer provides a variety of ergonomic designs to improve the safety of clinicians:
- Cuff Type:
Knit cuffs are used due to their comfort during prolonged procedures, whereas elastic cuffs provide a more secure seal to prevent the spread of infectious diseases in wards. Thumb-loop designs are prevalent among China Disposable Isolation Gowns Manufacturers to avoid sleeve ride-up during glove wearing.
- Closure Systems:
B2B customers have the option of purchasing neck ties, tape tabs, or hook-and-loop (Velcro) closures. The manufacturers of high-volume isolation gowns frequently recommend using tape tabs to make quick donning and doffing in emergency situations.
With the selection of an Isolation Gown Manufacturer China partner with a comprehensive grasp of these construction sciences, procurement teams are able to provide their frontline workers with the greatest protection possible in the 2025 market.
The 5-Stage Isolation Gown Manufacturing Process
For a professional procurement officer, the only way to really vet the quality of a supplier is to know the inner workings of an isolation gown manufacturer. One of the characteristics of an industry leader is a clear isolation gown production process.
At BKAMED, our Hubei facility follows a five-stage process designed to address the high-volume needs of global isolation gowns manufacturers’ markets, while maintaining the precision required in a surgical process.
Phase 1: Raw Material Synthesis & Testing
The journey of a high-quality garment begins with polymer science. A major disposable isolation manufacturer begins by obtaining high-purity polypropylene pellets. During this stage, the manufacturer of the isolation gown must check the purity and the melt flow index of the resin. Any contamination at this stage may create pinholes in the final fabric, which is a critical failure to covid isolation gowns manufacturers.
For B2B purchasers, the most important indicator here is the GSM (Grams per Square Meter) consistency. A trusted Isolation Gown Manufacturer China ally will conduct a batch test of raw fibers to verify that the density in the Spunbond and Meltblown layers is within the target AAMI protection levels.
Phase 2: Fabric Extrusion and Lamination (SMS Production)
This is the essence of isolation gown manufacturing. The polypropylene is melted and extruded into spinners to form Spunbond layers for strength and Meltblown layers to provide filtration. A leading company in the manufacturing of advanced isolation gowns uses an uninterrupted online lamination process to produce SMS, SMMS, or SSMMS fabrics.
In this step, a leading facility producing China isolation gowns applies automated optical sensors to identify any thinning in the Meltblown layer. This ensures that when a wholesaler orders from a disposable isolation gown manufacturer, each square inch of the cloth offers a consistent barrier against fluids and pathogens.
Phase 3: Precision Cutting and Assembly
After validation of the fabric rolls, they are transferred to the Conversion floor. In contemporary isolation gown production, automated CNC (Computer Numerical Control) machines are used instead of manual cutting. This guarantees that all gowns produced by the isolation gown manufacturer are mathematically flawless in design, minimizing material loss and standardizing the sizing between S and 4XL.
For blue isolation gown manufacturers, quality becomes bifurcated in the assembly approach. Whereas simple suppliers operate with conventional sewing, a leading Isolation Gown Manufacturer China partner, such as BKAMED, employs high-speed ultrasonic welding devices.
This technology joins the sleeves and body with high-frequency sound waves, thus removing needle holes that are present in the products of lesser isolation gown manufacturers. For China Disposable Isolation Gowns Manufacturers, this is a very important step towards attaining AAMI Level 3 certification.
Phase 4: Sterilization Methods (If Applicable)
Although a variety of isolation gowns are marketed as “non-sterile” to be used in the general ward setting, there are high-risk environments where a sterile disposable isolation gown manufacturer is needed.
During this stage, the isolation gown manufacturer exposes the clothing to either Ethylene Oxide (EO) gas or Gamma Irradiation. For B2B wholesalers, EO sterilization is the preferred choice since it highly penetrates the multi-layered SMS fabric without compromising the integrity of the polymer fibers.
A professional isolation gowns manufacturer will provide a Certificate of Sterilization with a proven Sterilization Assurance Level (SAL) of $10^{-6}$, which means that the product can be safely used in the most sensitive clinical settings.
Phase 5: Packaging and Quality Check (QC)
The last phase of the production of isolation gowns is dedicated to logistics that are wholesaler-ready. All gowns are checked in terms of tie strength, cuff, and consistency of seams. As a leading company in the manufacturing of China Disposable Isolation Gowns, BKAMED uses vacuum-packaging in bulk ordering. This decreases the physical size of the delivery, permitting B2B purchasers to stack a greater number of products into a 40ft High Cube box, significantly lowering the landed cost of each unit.
Then the manufacturer of isolation gowns marks each main pouch and each secondary carton with a different Batch Code and Expiry Date. It is this degree of traceability that makes the difference between a professional isolation gown manufacturer and a generic supplier, providing the transparency needed during hospital audits and regulatory compliance.
By mastering these five stages, an Isolation Gown Manufacturer China partner guarantees that all deliveries match the world’s expectations of the modern healthcare wholesalers.
Technical Specifications & Performance Metrics for an Isolation Gown Manufacturer
When it comes to a B2B procurement team, the only objective method of comparing two manufacturers of isolation gowns is through technical data. In the highly competitive isolation gown manufacturing, marketing assertions are not more important than verified laboratory measurements. To promote clinical safety, a professional isolation gown manufacturer needs to present documents that show their products comply with the ANSI/AAMI PB70 and ASTM F2407 standards.
ANSI/AAMI PB70 Standards Explained in Isolation Gown Manufacturing
The ANSI/AAMI PB70 standard is the most significant standard for barrier performance. Any disposable isolation gown manufacturer operating at an international scale has to expose their clothing to two leading tests:
- AATCC 42 (Water Resistance: Impact Penetration Test)
This is a test that determines the resistance of the fabric to water penetration impact. The water is sprayed onto the cloth, and a blotter paper is weighed to determine the amount of water that was absorbed. For a blue isolation gown manufacturer, achieving a result of $\leq 1.0g$ is the target for Level 2 and Level 3 protection.
- AATCC 127 (Water Resistance Hydrostatic Pressure Test)
This is a challenging test in which the fabric is placed under increasing water pressure. In isolation gown manufacturing, this identifies whether the material would hold when leaned over or sat upon during a procedure.
A top Isolation Gown Manufacturer in China, such as BKAMED, ensures that their Level 3 gowns can withstand at least 50cm of water pressure, which is a solid barrier against emergency department workers.
The technical guidelines from the Association of the Advancement of Medical Instrumentation (AAMI) note that such tests should be done on both the fabric and the essential seams to determine whether the isolation gowns manufacturer has created a truly impervious garment.
ASTM F2407: The Physical Performance Blueprint
Whereas AAMI deals with fluid barriers, ASTM F2407 is concerned with the physical integrity of the garment. In vetting a disposable isolation gown manufacturer, B2B buyers must demand data on:
- Tensile Strength (ASTM D5034): It guarantees that the gown does not tear when the person wearing it moves or reaches.
- Tear Resistance (ASTM D5587): This is used to evaluate whether the fabric can snag medical equipment.
- Seam Strength (ASTM D1683): This is critical to China Disposable Isolation Gowns manufacturers as the seams should be as strong as the fabric.
A reputable isolation gown manufacturer will incorporate such measurements in their technical data sheets to assure the wholesaler of the structural integrity of the garment.
Biocompatibility Standards (ISO 10993)
Since these garments are worn by medical personnel during 12-hour shifts, an isolation gown manufacturer needs to ensure that the materials are skin-safe. China’s isolation gown manufacturers that conform to ISO 10993 regulations test for cytotoxicity, sensitization, and skin irritation.
This is especially crucial for blue isolation gowns manufacturers that utilize special dyes or anti-static coating. For a disposable isolation gown manufacturer, a key factor for high-quality isolation gown manufacturing is ensuring that the polymer fibers are not allergenic.
Metric Cheat-Sheet for B2B Wholesalers
To make the procurement process easier, the following weight-to-protection guide will be useful when negotiating with an Isolation Gown Manufacturer China partner:
| Material Type | Typical GSM | Recommended AAMI Level | Best Use Case |
| Spunbond PP | 20–30 GSM | Level 1 | Visitor cover/Basic care |
| SMS/SMMS | 30–45 GSM | Level 2 | ICU/Phlebotomy/Suturing |
| High-Density SMS | 45–65 GSM | Level 3 | Emergency/Arterial blood draw |
| PE-Coated SMS | 50+ GSM | Level 4 | Infectious disease/Viral risk |
As a leading China Disposable Isolation Gowns Manufacturers authority, BKAMED offers all spectrums of these specifications.
Since COVID isolation gowns manufacturers scale the requirements, we suggest prioritizing 40-50 GSM SMS fabrics to strike a balance between barrier protection and high-stress breathability.
Through making such technical requirements, B2B customers can demand the best global standards of clinical excellence from their isolation gowns manufacturer.
Quality Control, Inspection, and Defect Prevention
Within the B2B medical supply chain, one delivery of faulty garments can destroy the reputation of a wholesaler and undermine the safety of frontline workers.
An international isolation gowns manufacturer does not simply respond to mistakes; they construct a structure in which the probability of flaws is reduced through strict adherence to “Total Quality Management” (TQM).
When auditing a disposable isolation gown manufacturer, procurement teams should not only investigate the showroom but also analyze the internal inspection rules of the manufacturer.
The Role of AQL (Acceptance Quality Limit) in Procurement
The AQL ( Acceptance Quality Limit ) is the industry standard used to vet a batch of China isolation gowns manufacturers. For medical-grade PPE, most high-volume isolation gowns manufacturers work under ISO 2859-1 standards, typically using an AQL of 1.5 or 2.5 for major defects.
- AQL 1.5: It’s commonly used with surgical-grade or AAMI Level 3 products. It implies that when over 1.5 percent of the sampled batch exhibits defects of major types, the whole lot is abandoned.
- AQL 2.5: More typical with Class I garments of a generic disposable isolation gown manufacturer.
B2B buyers are supposed to include their desired AQL level in the Purchase Order (PO) when negotiating with an Isolation Gown Manufacturer China partner. The lower the AQL, the stricter the inspection process and the greater the dedication of the isolation gowns manufacturers to quality.
Critical vs. Major Defects in Isolation Gown Manufacturing
A professional isolation gown manufacturer categorizes defects to focus on safety. When conducting a third-party inspection of a shipment of China Disposable Isolation Gowns Manufacturers, inspectors will examine:
- Critical Defects (Zero Tolerance): Contaminants that are needles, mold, or any biological contaminant. Any event leads to automatic batch rejection.
- Major Defects: Problems that undermine the barrier, including pinholes in the SMS tissue, unsealed seams, or lost ties. A seam failure is the most frequent significant flaw in the case of a blue isolation gowns manufacturer specialist.
- Minor Defects: These are aesthetic problems that do not affect safety, including loose threads (more than 1cm), slight color variations, or wrinkled packaging.
Batch Traceability and Lot Coding
The isolation gown manufacturing in the modern world demands digital responsibility. Each carton shipped out of a leading Isolation Gown Manufacturer China factory must include a Unique Device Identifier (UDI) or a comprehensive Lot Number. This enables the isolation gown manufacturer to determine the batch of raw material, the machine, and the QC team that accepted it.
For covid isolation gown manufacturers, this traceability is crucial to the management of recalls. If a particular batch of SMS fabric is discovered to be of lower quality, the disposable isolation gowns manufacturer can identify precisely which wholesalers received the goods, stopping a regional problem from becoming an international liability.
Third-Party Inspection Protocols
Though internal QC is necessary, professional B2B buyers typically contract third-party companies (such as SGS or QIMA) to conduct a Final Random Inspection (FRI) at the China isolation gowns manufacturer plant. Eurofins Assurance states that a typical FRI includes verifying the Product Appearance, Workmanship Quality, and Functionality Tests (including determining whether the thumb loops can break under tension).
Wholesaler Tip: It is always advisable to insist on a Pre-Shipment Inspection Report from your isolation gown manufacturer before paying the final bill. This guarantees that the output of the isolation gown manufacturing matches the gold sample, which was approved in the tender stage.
By collaborating with a China Disposable Isolation Gowns Manufacturers leader that embraces third-party audits and a standard of AQL 1.5, B2B organizations can keep their supply chain robust, compliant, and, above all, safe to the end-user.
Global Compliance, Certifications, and Regulatory Pathways
The isolation gown manufacturer in the B2B industry can be as trustworthy as their compliance portfolio. For procurement managers, the most confusing part of sourcing is moving through the maze of international regulations. Whether you are importing from an Isolation Gown Manufacturer China or a domestic supplier, you must ensure that the product complies with the specific legal frameworks of the target market.
The North American Market: FDA and 510(k) Clearance
In the United States, the FDA regulates medical gowns under Class I or Class II medical devices.
- Class I (Exempt): The majority of Level 1 and Level 2 gowns produced by a disposable gown manufacturer fall under this classification. They are not subject to a 510(k) premarket notification but have to be manufactured in an FDA-registered plant.
- Class II (510(k) Required): All gowns that contain the term Surgical, Surgical Isolation, or are rated at AAMI Level 3 or 4 are Class II devices. A professional isolation gown manufacturer should submit a 510(k) number of clearance (usually with K at the beginning of the number) in order to demonstrate that the device is safe and effective.
With active FDA registrations as a leading Isolation Gown Manufacturer China partner, BKAMED has ensured that wholesalers avoid the “red tape” that many China Disposable Isolation Gowns Manufacturers have been known to have.
The European Market: MDR and EN 13795:2025
As of 2025, the European Union has introduced the last stage of the Medical Device Regulation (MDR 2017/745). To sell a disposable isolation gown in the EU, a manufacturer of this product must meet the recently revised harmonized standard EN 13795-1:2025. These standards have lately added stiffer quartile-based statistical assessments for liquid resistance.
A CE Mark and valid Declaration of Conformity (DoC) should be sought when vetting China isolation gowns manufacturers. For B2B purchasers in the UK, the UKCA marking is currently compulsory, and the isolation gowns manufacturer must comply with the technical requirements, which are largely the same as those of the EU MDR but regulated by the MHRA.
ISO 13485: The Gold Standard for Isolation Gown Manufacturing
All isolation gowns manufacturers should seek to get the most significant certification, which is ISO 13485:2016. Contrary to a generic ISO 9001 quality mark, ISO 13485 is specific to the medical device industry. It requires the isolation gown manufacturing process to include:
- Risk Management: Researched examination of the likely failure points within the barrier of the gown.
- Sterile Assurance: If the disposable isolation gown manufacturer is selling sterile products, they should have verified EO ( Ethylene Oxide ) or Gamma cycles.
- Environmental Control: Evidence that the isolation gowns manufacturer is in a clean, controlled environment to avoid the contamination of bioburden.
Checklist for B2B Compliance Vetting
Before signing an agreement with a China Disposable Isolation Gowns Manufacturers partner, demand a Compliance Pack that includes:
- FDA 510(k) Summary (Level 3/4, or Surgical Gowns).
- ISO 13485:2016 Certificate by a certified body such as TUV or SGS.
- Third-party Test Reports for ASTM F2407 and AAMI PB70:2022.
- CE/UKCA Certificates containing valid Notified Body numbers.
By prioritizing an Isolation Gown Manufacturer China leader who invests in such certifications, B2B organizations safeguard themselves against customs confiscations and legal claims, and in addition, they guarantee that clinical staff are safeguarded by covid isolation gowns manufacturers that adhere to the highest international safety standards.
B2B Sourcing Strategy: Choosing the Right Partner
An isolation gown manufacturer is not just a supplier in the medical supply chain, but a clinical risk management partner. As of 2025, procurement has moved towards a resilience-first and not just-in-time focus. For B2B wholesalers, it is necessary to take a thorough look at technical capacity, logistical transparency, and customization flexibility to find the correct Isolation Gown Manufacturer China.
Evaluating Manufacturer Capacity and Lead Times
In auditing an isolation gown manufacturer, you have to differentiate between Standard Output and Surge Capacity.
- Daily Production: A leading disposable isolation gowns manufacturer must have a daily output of at least 100,000 to 150,000 units. This guarantees them the ability to meet multi-container contracts without overloading production lines.
- Emergency Preparedness: For covid isolation gowns manufacturers, flexibility in manufacturing during a pandemic is essential. Ask whether the factory has a Buffer Stock of raw SMS fabric. An effective partner in Isolation Gown Manufacturer China must be capable of doubling the production in 14 days with the activation of secondary cleanroom modules.
- Lead Times: The usual lead time for a 40ft HQ container (around 80,000-100,000 gowns) is between 20 and 30 days. Any isolation gown manufacturers offering much lower quotes might be losing the 24-hour out-gassing time necessary following EO sterilization.
OEM/ODM Customization Capabilities
A one-stop isolation gowns manufacturer enables B2B customers to customize products to meet market segments. Being one of the top China isolation gowns manufacturers authority, BKAMED provides a wide range of customization:
- Private Labeling: Have the name of your company printed on the chest or the outer-packaging of the gown in order to enhance your brand presence.
- Long Sizing: Although most manufacturers of disposable isolation gowns limit themselves to XL, there is an increasing demand for XXL to 4XL (Bariatric clothes). Make sure your partner can change patterns to offer 360-degree coverage for all body types.
- Premium Benefits: Customization, such as thumb-loop wrists to secure the glove, or over-the-head designs with higher donning speed, are premium features that make the difference between high-quality blue isolation gowns manufacturers and generic vendors.
Supplier Audit Checklist for Procurement Managers
Before entering into a long-term agreement with a China Disposable Isolation Gowns Manufacturers company, a physical or virtual audit is compulsory. Focus on these three pillars:
- Machinery Age & Maintenance: Does the manufacturer of the isolation gowns have modern ultrasonic welding machines (not older than 2022), or does it still rely on manual sewing? The modern machines minimize the factor of Human Error in seam integrity.
- Factory Condition: The manufacturing zone of the isolation gowns should be a certified Class 100,000 (ISO 8) cleanroom to avoid contamination of air.
- Labor Practices: Check whether the manufacturer of the isolation gowns follows the international labor regulations (BSCI or Sedex). Government tenders and huge hospital GPOs now have ethical sourcing as a mandatory condition.
Pricing Structures in Bulk Procurement
The knowledge of Incoterms is essential to determine the Landed Cost of your shipment.
- FOB (Free On Board): The most used by seasoned B2B buyers. The isolation gown manufacturer transports the item to a port (such as Shanghai or Wuhan). The freight and insurance are at your control, providing superior visibility and possible savings in the case you have a trusted freight forwarder.
- CIF (Cost, Insurance, and Freight): The disposable isolation gown manufacturer takes care of the goods until they are at your destination port. This is hassle-free and has a tendency to carry an undisclosed premium on the shipping rates.
When dealing with large-volume China Disposable Isolation Gowns Manufacturers deals, we suggest FOB. This enables you to take advantage of the efficiencies of vacuum-packaging to be able to put more units in a container and reduce your shipping cost per unit directly.
Logistics, Supply Chain, and Inventory Management
For a B2B wholesaler, the interaction with a manufacturer of isolation gowns does not cease once the goods leave the factory floor.
It is the efficient logistics and careful keeping of inventory that will turn a bulk purchase into a successful, high-availability supply chain.
The core competency of any medical procurement professional in 2025 is the capacity to optimize container loads and the integrity of fabrics during long-term storage.
MOQs and Economies of Scale: Optimizing the Payload
Minimum Order Quantity (MOQ) established by an isolation gown manufacturer is directly related to the economics of isolation gown manufacturing. For a major Isolation Gown Manufacturer China partner, MOQs are usually designed with the conventional shipping container volumes in order to optimize cost-effectiveness.
- 20ft Container (FCL): This can typically accommodate approximately 35,000-45,000 isolation gowns, depending on the GSM and folding style. This suits well with regional distributors or special clinics.
- The 40ft High Cube (HC) Container: The industry standard of global wholesalers. A high-tech disposable isolation gown manufacturer that uses a vacuum-compression package can accommodate 80,000-100,000 units within a 40ft HC.
By selecting a China Disposable Isolation Gowns Manufacturers partner who understands high-density packing, B2B buyers can save up to 25 percent of their per-unit shipping expense. This Logistical Alpha becomes essential in case of competing on thin margins in the isolation gowns manufacturer wholesale market.
Storage and Shelf-Life Management
Medical gowns do not last forever. The multi-layer SMS fabric created in the process of isolation gowns manufacturing is prone to degradation when exposed to unfavorable environmental conditions. A typical professional isolation gowns manufacturer will certify a 3 to 5-year shelf life of non-sterile gowns, provided they are stored according to protocols.
Best Practices for Inventory Longevity:
- Temperature: Keep a cool, dry place (preferably 15 °C to 30 °C). Temperature may disrupt the integrity of the polypropylene of a product for a disposable isolation gown manufacturer.
- Humidity Control: For the relative humidity, it should be less than 60%. Too much moisture can damage the cardboard secondary packaging and even support the growth of microorganisms, even in non-sterile batches from China isolation gowns manufacturers.
- Light Protection: Excessive UV-radiation may destroy polymer bonds in the fabrics of blue isolation gown manufacturers, resulting in loss of tensile strength and barrier efficiency.
- FEFO (First-Expired-First-Out) Rotation: The use of a FEFO system guarantees that gowns are allocated according to the expiry date of a particular batch and not according to their time of arrival. The practice not only avoids aging-out of inventory but also ensures that clinicians are supplied with products with a proven barrier integrity.
- Physical Load and Stacking Limits: Following the highest stacking height that is prescribed by the manufacturer helps avert overpressure that may cause the meltblown filtration layers to be trampled down. Preservation of the structural form is so that the gown does not lose its rated AAMI protection, and that the protective packaging covers are not damaged.
Procurement managers ought to make sure that their isolation gown manufacturer gives clear dates of “Expiration dates or Re-test dates on each master carton, as it’s now a strict requirement by both the FDA and the CE regulators in 2025.
Mitigating Global Supply Chain Risks: The “China Plus One” Strategy
Although an Isolation Gown Manufacturer China is the most affordable option as the primary source, modern resilience strategies suggest diversification as a measure. China Plus One model implies that 70-80 percent of the volume should be sourced by the leader of the China isolation gowns manufacturer industry, such as BKAMED, but the supply chain should be supported by a backup location in regions like Southeast Asia or local warehousing.
The Role of Local Warehousing: To counter the instability of ocean shipping, most B2B buyers now demand that their isolation gowns manufacturer to fund “Safety Stock” programs. Even a leading disposable isolation gown manufacturer can have regional distribution centers in the US or Europe. This gives the wholesalers an opportunity to utilize local stock in case of shipping delays, so that hospitals never get into the so-called stock-out situation.
Moreover, in the case of COVID isolation gowns manufacturers who are focused on responding to emergencies, a Live Inventory connection between the ERP of the isolation gown manufacturer and the wholesaler system is necessary. This digital transparency enables real-time demand predictions, guaranteeing that the isolation gown manufacturing schedule is always in sync with the actual burn rate of the healthcare system.
With these logistical subtleties, B2B organizations ensure that the high-quality products they purchase from an Isolation Gown Manufacturer China partner arrive on time, in excellent shape, and at the minimal landed cost achievable.
Future Trends and Sustainability in Gown Manufacturing
The healthcare business in the world is taking a paradigm shift of monumental proportions. In 2025, the discussion of the isolation gown manufacturer has shifted to the topic of Value-Based Manufacturing as opposed to the mere number of units.
With increasing environmental regulation and more sophisticated clinical demands, the future of isolation gown manufacturing is currently being shaped by three pillars: environmental circularity, intelligent bio-defensive material, and AI-driven inventory logistics.
1. Biodegradable Materials: The End of the “Plastic Crisis” in PPE
The amount of medical waste produced annually, estimated at more than 200,000 tons in terms of gowns alone, can no longer keep up. In reaction, the major Isolation Gown Manufacturer China industry is turning to bio-based polymers.
- Polylactic Acid (PLA) Gowns: PLA non-wovens are derived from renewable materials such as corn starch or sugarcane and are the most promising alternative to conventional polypropylene. Unlike synthetic gowns, which take centuries to break down in landfills, PLA-based items from a forward-thinking disposable isolation gown manufacturer can break down under industrial composting conditions in several months.
- Reduced Plastic Packaging: Future-oriented China Disposable Isolation Gowns Manufacturers are also redesigning the shipping footprint. These can involve the substitution of single plastic pouches with biodegradable films or the use of bulk-folded “interleaved” dispensing boxes that reduce the primary plastic waste by 40 percent per container.
- The Circularity Challenge: Although biodegradable gowns can minimize solid waste, the latest research from institutions such as Cornell University indicates that end-of-life management plays a vital role. The future of the isolation gown manufacturer will probably collaborate with waste-to-energy plants to ensure that the methane generated during the biodegradation process is captured and recycled.
2. Smart Gowns: Integrating IoT and Real-Time Monitoring
We are moving into the world of the “Connected Clinician”. A high-tech isolation gowns manufacturer is no longer just selling a piece of clothing; they are selling a data point.
- The RFID-Enabled Inventory: A disposable isolation gown manufacturer can equip the collar or hem with passive, flexible RFID (Radio Frequency Identification) tags and enable the hospital to automate inventory management. Smart cabinets are now able to record the precise number of available gowns and automatically send a replenishment order to the Isolation Gown Manufacturer China when the levels reach a critical point.
- Compliance Tracking: Smart gowns can be used to monitor the “Donning and Doffing” cycles in high-risk infectious disease units. Exit sensors in isolation rooms can also be used to detect whether a nurse is wearing a gown to enforce infection control measures and minimize the risk of Hospital-Acquired Infections (HAIs).
3. Anti-Microbial Coatings: The Active Barrier
Conventional gowns are passive barriers, meaning they merely seal fluids. The upcoming isolation gown manufacturers are creating an “Active Barriers” that deactivate pathogens on contact.
- Silver-Ion (Ag+) Impregnation: Silver nanoparticles are being incorporated in the SMS layers during the isolation gown manufacturing process. These ions interfere with the microbial cell membrane to offer a log- kill rate of up to 99.9 percent on bacteria and some viruses.
- Zinc-Based Nanostructures (ZnO): Zinc oxide is also becoming a favorite choice among blue isolation gowns manufacturers since it is Generally Regarded as Safe (GRAS) by the FDA. ZnO coating offers additional antimicrobial protection along with high photocatalytic functionality, making the gown unpleasant to the survival of viruses even in low-light clinical conditions.
- Nanotechnology Integration: More than 18 major China isolation gowns manufacturers are already developing nanotechnology to improve the liquid-repellency of Level 3 gowns, allowing fluids to bead off in a more efficient way than before.
4. Advanced Ergonomics and Thermal Management
In 2025, worker burnout is a major issue. An isolation gown manufacturer that does not consider comfort will lose market share.
- Radiative Cooling Fabrics: The development of new composite fabrics is underway that reflect 87% of the thermal radiation, keeping the wearer up to 9.0 °C cooler than the usual SMS gowns.
- Moisture-Wicking Layers: With the addition of a hydrophilic inner layer to the trilaminate SMS structure, the isolation gown manufacturing process can now create gowns that can push sweat off the skin of the clinician; a very important aspect of extended operations in hot conditions.
5. Robotics and AI-Driven Automation: The “Zero-Touch” Factory
The major change in isolation gown manufacturing in 2025 is the replacement of the labour-intensive assembly with a fully automated production unit.
- Robotic Ultrasonic Welding: Seams have been a source of human error. Modern China isolation gown manufacturers are currently using 6-axis robotic arms with rotary ultrasonic horns. These robots can follow intricate gown profiles with sub-millimeter accuracy, a feat that makes sure AAMI Level 3 seals are 100 percent reproducible on a one-million-unit batch.
- AI Computer Vision in QC: The high-speed cameras built into the production line are now scanning all square inches of the SMS fabric in real-time using artificial intelligence. These systems are able to identify the micro-pinholes or thinning in the meltblown layer that the human eye cannot see, and automatically adopt and eliminate defective garments before they get to the packaging process.
- Onshoring with Automation: This trend is also enabling “near-shoring.” With less dependency on manual labor, an isolation gown manufacturer can now manufacture cost-effective incentives that are nearer to North American and European markets, radically reducing the supply chain of emergency PPE.
6. Advanced Thermal Management: Radiative Cooling and RET Optimization
Hospital GPOs are concerned with clinician fatigue caused by heat stress. The new generation of isolation gown manufacturing is centered on the physics of thermodynamics.
- Radiative Cooling Textile: An emerging technology of radiative cooling textiles uses the composite SMS fabrics designed to reflect up to 90% of solar and ambient thermal radiations while at the same time releasing the wearer’s body heat to the atmosphere. This technology is able to maintain a healthcare practitioner up to 5 °C- 9 °C lower than standard polypropylene.
Low-RET (Resistance to Evaporative Heat Transfer) Materials: An ideal disposable isolation gown manufacturer can achieve a RET value of less than 6 by optimizing the fiber diameter of the laid-up spunbond layers. This means highly breathable clothing that lets the sweat vapor out quickly without damaging the liquid barrier- an essential innovation in the field of surgeons and nurses working in high-intensity, multi-hour operations.
The 2025 Outlook for B2B Procurement
As a B2B wholesaler, you want to identify an Isolation Gown Manufacturer China ally that is making active investments in these Future-Tech segments. Companies like BKAMED are already incorporating these innovations into their product roadmaps for 2026.
When you purchase a product made by a top disposable isolation gown manufacturer that nurtures its social responsibility alongside its focus on Smart Protection, you are not only purchasing a product, but you are insuring your business against changing regulatory policies and the growing need of the world to have ethical, technology-driven medical products in the future.
Conclusion
As 2025 healthcare procurement moves into a complex environment, technical diligence is no longer a mere best practice, but a requirement of clinical safety and institutional liability.
As we have examined, the distinction between an ordinary garment and a high-performance barrier is found in the microscopic fineness: the integrity of an ultrasonic weld, the accuracy of the GSM of a meltblown layer, and the strict compliance with the requirements of AAMI and ISO 13485.
In the case of B2B wholesalers, the price of a single low-quality batch is much higher than the potential marginal benefits of unverified suppliers.
Selecting the appropriate isolation gown manufacturer implies selecting a partner that is totally transparent in all their manufacturing process phases.
BKAMED (Hubei Baikang Medical Instrument Co., Ltd) is the leader in this business with more than 30 years of manufacturing excellence and with state-of-the-art automation and a global compliance portfolio.
With a focus on technical accuracy, sustainable innovation, and ethical supply chain, BKAMED is looking forward to supplying healthcare providers with the absolute best in protective technology.
Are you ready to secure a supply chain that never compromises on clinician safety?
Your procurement strategy should not be random in times of global health requirements that are unpredictable. By collaborating with BKAMED, you are no longer dealing with transactional purchases; you enter into a partnership characterised by technical superiority and local trustworthiness.
Why B2B Wholesalers Choose BKAMED:
- Decades of Experience: Build on 30 years of specialized expertise in manufacturing medical-grade textiles.
- Unrivaled Conformance: Gain entry to a portfolio with full compliance with ISO 13485, CE, and FDA certifications, which will allow a smooth transition to international markets.
- High-Volume scalability: We can scale up to 100,000 units of vertical integration of SMS fabric production daily, and we can deliver new orders instantly.
- Designed for Your Brand: Whether it is private labeling, industry-specific bariatric fitment, or vacuum-compressed packaging, we will customize each of your orders to your unique logistics and branding requirements.
Take the next step in professional Procurement. Do not leave your barrier protection to chance. Become one of the hundreds of healthcare distributors in more than 70 countries who rely on BKAMED for their high-compliance sourcing.
Contact our B2B Sales Team Today or order a Technical Sample Kit today to see the BKAMED difference first.

