Wholesale EO Sterile Uncuffed Endotracheal Tube – PVC, Graduated Depth Control, Single-Use Airway Management
Wholesale EO Sterile Uncuffed ET Tubes for pediatric/specialized use. Thermoplastic PVC, X-Ray visibility, dual-marked size, and Murphy eye safety. Ready for rapid intubation.
Description
Wholesale EO Sterile Uncuffed Endotracheal Tube – Precision Pediatric Airway
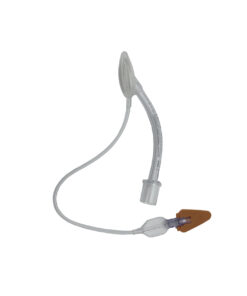
The Uncuffed Endotracheal Tube is a highly specialized, single-use airway device mandated for specific clinical applications, particularly in the intubation of neonates and small children. Its design is rooted in the traditional anatomical understanding that the cricoid ring, not the vocal cords, is the narrowest point in the pediatric airway. The use of an uncuffed design prevents direct pressure necrosis on the delicate subglottic mucosa, minimizing the risk of complications such as post-extubation stridor or subglottic stenosis. As a trusted wholesale supplier, BKA MED provides this essential PVC tube, manufactured to the highest standards of safety and dimensional precision, ensuring effective device insertion into the trachea to prevent airway obstruction and respiratory failure.
Addressing Clinical Challenges with Precision Features
The primary challenge associated with uncuffed tubes is the potential for gas leakage around the tube, which can compromise ventilation, lead to inaccurate monitoring (ETCO_2), and allow the escape of anesthetic gases. Our Uncuffed Endotracheal Tube is engineered with specific features to maximize performance and mitigate these issues, providing a reliable and safe seal at optimal tracheal pressure:
Advanced Safety and Positional Control
- Thermoplastic PVC Construction: The choice of thermoplastic PVC ensures a tube with high elasticity. This material performs optimally, remaining sufficiently firm at room temperature for smooth, controlled insertion, yet softening slightly at body temperature to conform closely to the tracheal lumen. This adaptability helps achieve the ‘minimal leak technique’ crucial for uncuffed systems.
- Dual Positional Verification: Precision is non-negotiable in airway management. Our tube integrates two layers of positional assurance:
- Full-Length X-Ray Marker: The radio-opaque line running over the entire tube length provides definitive, real-time confirmation of the tube tip’s exact location via fluoroscopy or X-ray.
- Graduated Depth Markers: The clear markings graduated every 2cm provide immediate visual feedback. These depth markers allowing for the tube position control are essential for noting the insertion depth at the teeth or lips and quickly detecting any subsequent dislodgement.
- Integrated Murphy Eye Safety: The tube is equipped with a side Murphy eye, offering a critical secondary path for airflow. This design prevents acute airway occlusion if the bevel of the distal tip becomes lodged against the tracheal wall or blocked by secretions, ensuring continuous ventilation capability.
Sterility, Standardization, and Wholesale Procurement
Guaranteed Hygiene and Single-Use Protocol
For critical devices like the ET tube, a strict single-use protocol is vital for patient safety and infection control:
- EO Sterilized: Every unit is fully EO sterilized (Ethylene Oxide), a reliable process that ensures the product is biologically safe and ready for immediate use upon opening.
- Single-Use Design: The tube is designated for single-use, eliminating the risks, quality control demands, and logistical burden associated with cleaning, inspection, and sterilization required for reusable equipment.
- Individual Packaging: Each tube is protected within individual paper-foil packaging, maintaining a sealed, sterile environment until required at the bedside or in the operating theatre.
Universal Compatibility and Identification
- Standard Connector: The tube features a standardized Ø 15mm connector, guaranteeing universal compatibility with all modern anesthesia systems, mechanical ventilators, and manual resuscitation equipment. The transparent nature of the connector allowing for visual control of gas flow and moisture build-up.
- Clear Size Marking: The tube size is clearly marked in two places (on the connector and on the tube), minimizing the risk of selecting the incorrect size during time-sensitive intubation procedures. This dual marking system is a best practice requirement for institutional safety protocols.
For distributors and healthcare providers managing large inventories of pediatric and specialized airway equipment, securing a continuous supply of this high-specification Wholesale EO Sterile Uncuffed Endotracheal Tube is crucial. Its design directly supports best clinical practice, balancing the need for airway patency with the minimal risk of mucosal injury.




Reviews
There are no reviews yet.