Wholesale Pen Type IV Cannula – Safety Disposal Packaging, PTFE/PUR/FEP Catheters, High Flow 14G to 26G
Pen Type IV Cannula (No Wings/Port). Safety Disposal Pack, Non-Pyrogenic, USP Class VI compliant PTFE catheter. Full range 14G to 26G.
Description
Wholesale Pen Type IV Cannula Needle Safety Disposal – Advanced Catheter Materials and Needle-Stick Prevention
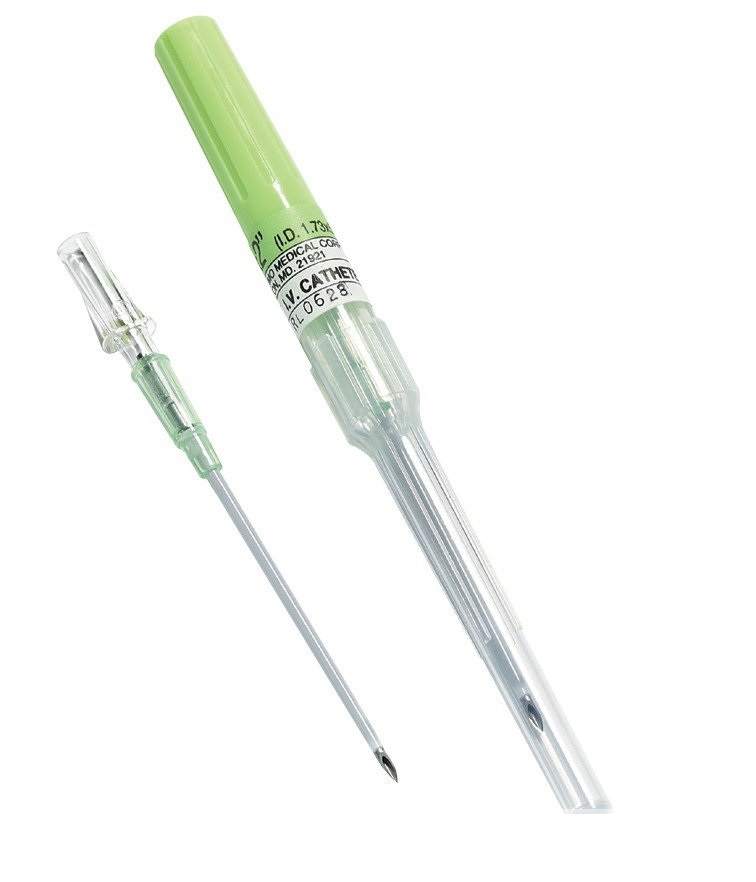
The Disposable IV Cannula Without Wings Without Port in the Pen Type configuration is a high-performance vascular access device optimized for general intravenous, fluid replacement, and medication delivery procedures. Unlike cannulas with additional features like wings or injection ports, the straight-hub, wingless, port-less design simplifies insertion and is favored by clinicians for procedures where quick, uncomplicated access is prioritized. This product is defined by its advanced catheter materials, its compliance with USP Class VI standards, and its groundbreaking special pen-type packaging for safety disposal.
Safety-Integrated Packaging and Aseptic Handling
The most significant differentiator for this IV Cannula line is the packaging, which elevates user safety and waste management standards:
- Special Pen-Type Safety Disposal: The design of the primary packaging unit is engineered to facilitate immediate, safe disposal of the sharp, contaminated device. The special pen-type packing is designed to completely enclose the used device to avoid contamination. This integrated feature helps minimize the risk of accidental needlestick injuries immediately after use, contributing significantly to occupational safety compliance and reducing institutional liability.
- Sterility Assurance: The device is manufactured to be Non-pyrogenic and is Sterilized by Ethylene Oxide (EO). This process guarantees a high Sterility Assurance Level (SAL), ensuring the device is safe for insertion into the patient’s vasculature.
- Aseptic Hub Technology: The hub is equipped with a Hydrophobic filter paper. This filter prevents blood and other fluids from escaping the catheter body during venipuncture and aspiration, while simultaneously allowing air to pass through, which is essential for proper flashback observation.
Optimized Catheter Materials and Flow Dynamics
The longevity and success of an IV catheter are dependent on the material and flow characteristics. This product offers multiple high-performance material options:
- Advanced Catheter Materials: The thin-walled catheters are available in high-grade polymers: PTFE (Polytetrafluoroethylene), PUR (Polyurethane), FEP (Fluorinated Ethylene Propylene), or ETFE (Ethylene Tetrafluoroethylene). These materials are known for their exceptional biocompatibility and low friction coefficient, which ensures smooth insertion and minimal risk of phlebitis or catheter-related infections. Radio-opaque versions are also available, making the catheter visible under X-ray for verification of placement.
- USP Class VI Compliance: The use of these materials ensures USP standards Class VI compliance. This certification is a critical assurance for medical procurement, indicating the materials have passed rigorous testing for safety and toxicity, confirming their suitability for direct contact with the human body.
- Tapered Tip Design: The gently tapered catheter tip aligns with the needle bevel to provide a smooth transition from needle to catheter. This feature, known as “flashback,” minimizes the force needed for insertion, reducing patient discomfort and the risk of trauma to the vessel wall.




Reviews
There are no reviews yet.